Pre-Fontan Procedures
Fontan palliation is the final palliation for the different forms of univentricular heart. This procedure is usually performed at the age of 2-3 years and the majority of children will need one or more surgeries before reaching this last step.
Although palliative surgeries are often considered to be technically simpler procedures than most reconstructive surgeries, the importance of performing them accurately should not be underestimated. Indeed, a technically imperfect execution can lead to complications that will decrease the chances of success of the following steps.
Read morePulmonary artery banding has been recommended for patients with a univentricular AV connection without pulmonary stenosis (as example: tricuspid atresia with transposition of the great arteries and a large VSD). Congestive heart failure, excessive pulmonary blood flow and pulmonary hypertension should prompt pulmonary artery banding before the age of 6 months to prevent the development of pulmonary vascular obstructive disease. Pulmonary banding is not a complex surgery in itself. The surgical risk stems mainly, as in the construction of a systemic-to-pulmonary artery shunt, from the significant physiological changes that the child undergoes at the time of surgery. The main technical difficulty is to determine the optimal diameter of the pulmonary artery. A small change in diameter can have a very pronounced effect on resistance and flow.
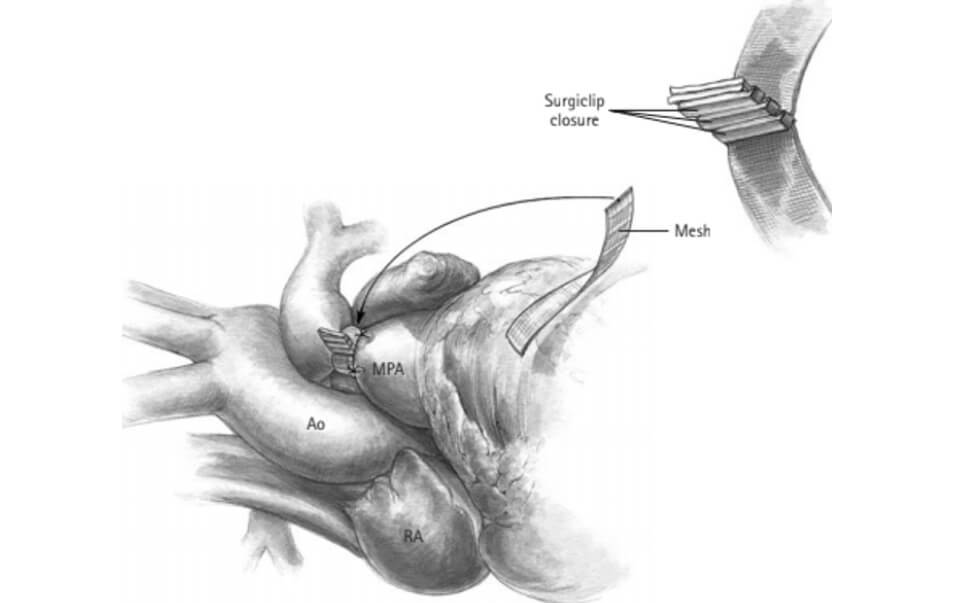
The surgical approach is normally done by median sternotomy. Limited dissection is performed to circumferentially release the main pulmonary artery. A strip of polyester or polytetrafluoroethylene of appropriate width (2 mm to 4 mm) for the size of the patient and his vasculature is selected. The optimal circumference of the banding is measured, and the strip is trimmed. The strip is then passed around the pulmonary artery and attached. It is important to position it distal at the sino-tubular junction so as not to cause a distortion of the pulmonary valve while being very careful not to pinch the right or left pulmonary arteries. The strip is attached to the weed of the pulmonary artery to prevent its migration. (Figure 1)
The banding of the pulmonary artery is adjusted during the procedure according to specific hemodynamic parameters. When encircling is applied, systemic blood pressure is expected to increase by approximately 10-20 mmHg. For patients who will ultimately have a Fontan palliation, the protection of the pulmonary vascular bed is paramount. The goal of the banding is to reduce the pulmonary blood flow enough to ensure a normal pulmonary pressure as even mild elevations of pulmonary vascular resistance (3U/m2) will preclude successful Fontan operation, this corresponds to a saturation of 80 to 85%. It is necessary to keep some leeway since the oxygen saturation will gradually decrease with the growth of the child; encircling gradually becoming proportionally tighter. Some patients with univentricular AV connections, after a pulmonary artery banding, will develop subaortic stenoses.
If the pulmonary blood flow is insufficient and the patient presents significant cyanosis, a systemic-to- pulmonary artery shunt can be proposed. These interventions allow communication between the systemic arterial and the pulmonary arterial networks. Their main objective is to increase the ratio of Qp/Qs, thus allowing the growth of the pulmonary arteries.
In 1945, Blalock, Thomas and Taussig performed the first systemic-pulmonary anastomosis in a tetralogy of Fallot by anastomosing the subclavian artery to the ipsilateral pulmonary artery, taking the name Blalock- Thomas-Taussig shunt (2). This was then a major advance for the treatment of cyanogenic heart diseases such as tetralogy of Fallot.
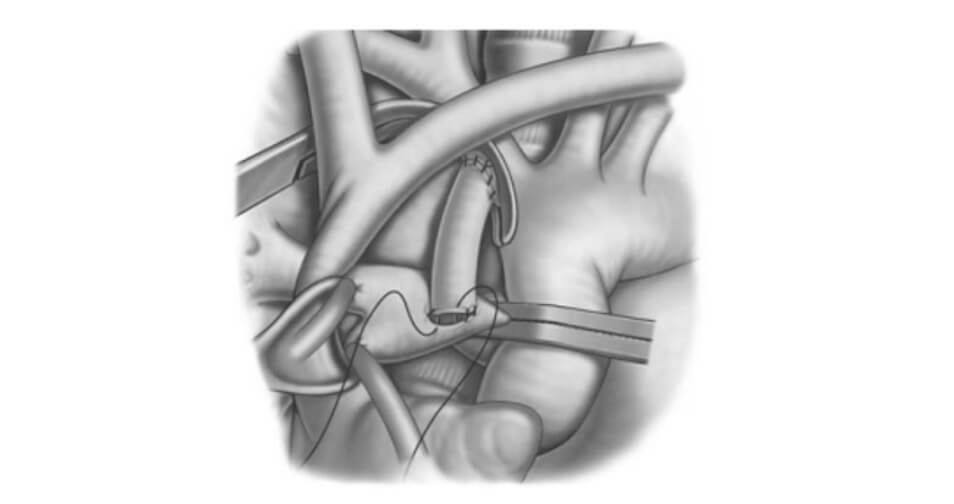
In 1981, De leval modified the procedure by interposing a prosthetic Gore- Tex tube between the subclavian artery and the pulmonary artery, allowing better control of the length of the shunt and the diameter of the tube to best calibrate the pulmonary flow during the neonatal period (Figure 2). This shunt can be achieved by thoracotomy from the 4th intercostal space or by sternotomy. The sternotomy approach is now favored for its technical simplicity, for the ability to ligate the ductus arteriosus and finally for its low risk of distortion and stenosis of the pulmonary arteries. Various studies have found an improvement in the long-term permeability of shunts by this approach (3). This anastomosis, commonly known as modified Blalock-Thomas-Taussig shunt, can be created with or without the use of cardiopulmonary bypass (CPB). It is often desirable to avoid CPB because of its inflammatory complications as well the need of a complete anticoagulation. However, it is sometimes impossible to create anastomosis without the support of the CPB due to anatomical or physiological considerations specific to the patients operated on. Under CPB and by sternotomy, a plasty of the pulmonary arteries is possible to increase, for example, a narrowed left pulmonary artery at the ductus arteriosus insertion site. The sternotomy approach is therefore more versatile and often preferred by surgeons.
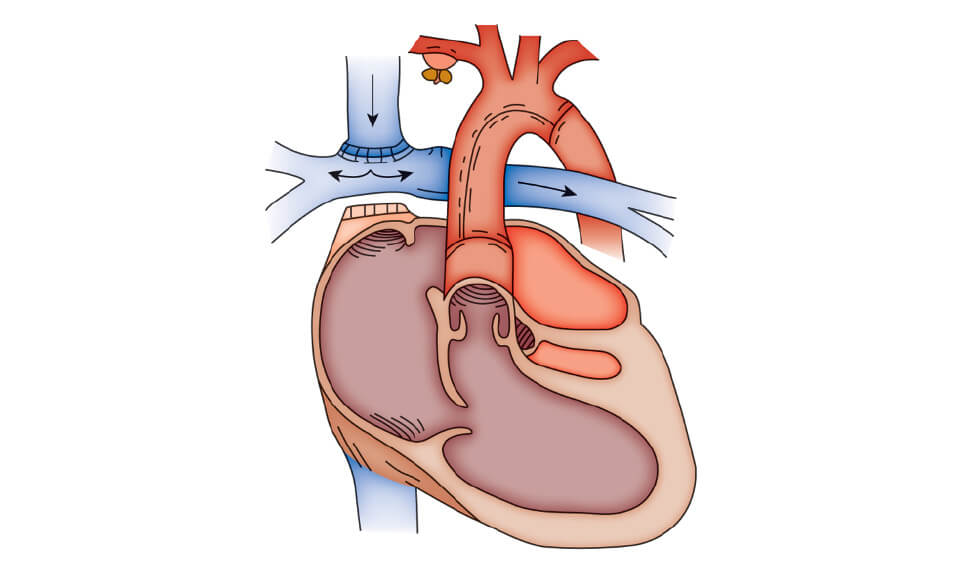
Beyond the first 4 to 6 months of life, the bidirectional cavopulmonary connection (bidirectional Glenn shunt) is an effective means of providing medium-term palliation for patients with univerntricular connection. The bidirectional cavopulmonary shunt consists of the anastomosis of the cardiac end of the superior vena cava to the right pulmonary artery leaving the pulmonary arteries confluent, this shunt provides effective pulmonary blood flow by directing desaturated blood directly into the pulmonary circuit. It does not increase the ventricular volume load as does a sytemic-to-pulmonary artery shunt. This technique has now replaced the classic Glenn shunt, consisting of anastomosing the superior vena cava to the right pulmonary artery resulting in nonconfluent pulmonary arteries. Serious complications such as the development of pulmonary arteriovenous fistulae around 5 years after placement of the Glenn shunt justify why this technique fell into disuse. The bidirectional cavopulmonary shunt produces excellent palliation in the first 2 to 3 years of life, progressive cyanosis and secondary erythrocytosis usually prompt further palliation such as the Fontan anastomosis.
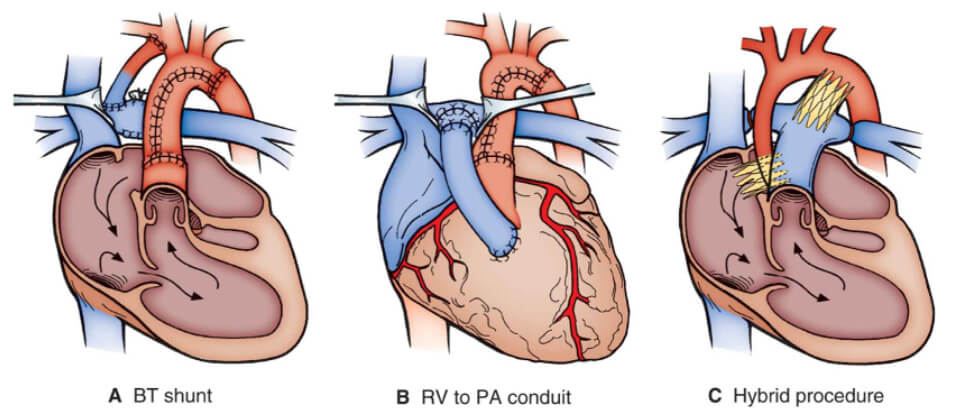
A staged surgical approach usually in 3 steps is now the recognized surgical approach to the palliation of patients with hypoplastic left heart syndrome. This approach ultimately leads the patient on a pathway to a single-ventricle in-series circulation usually culminating in a Fontan operation with the final result similar to patients with hypoplastic right heart syndrome. Most commonly stage 1 palliation consists of reconstruction of the aortic arch in to the right ventricular outflow, separation of branch pulmonary arteries from the right ventricle, and creation of a restrictive source of pulmonary blood flow form a systemic artery or directly from the single ventricle. Stage 2 unloads the single ventricle by replacing the systemic-to pulmonary artery shunt with a cavopulmonary anastomosis. (see above) The 3rd step is the completion of the Fontan anastomosis with or without fenestration.
The goals of stage 1 palliation include relief of ductal-dependent systemic flow, unrestriction of coronary artery flow, creation of a nonrestrictive atrial septal defect to prevent pulmonary venous congestion and provision of a reliable but restricted source of pulmonary blood flow (figure 4). Stage 1 palliation using either a Blalock-Thomas-Taussig shunt (figure 4A) or right ventricle-to-pulmonary artery conduit (Sano modification) (figure 4B) is done using CPB, deep hypothermia, and altered perfusion – either circulatory arrest or regional perfusion. The heart outflow is restructured via the pulmonary root with relief of coarctation and arch hypoplasia. The aim of stage 1 palliation is to create a stable anatomy that permits growth of the pulmonary bed so that it can accommodate subsequent single-ventricle palliation. The importance of a well performed stage 1 palliation should not be emphasized enough as recurrent and/or residual lesions are a source of interstage mortality and can preclude final palliation. The right ventricle-pulmonary artery conduit as a source of pulmonary blood flow has the theoretical advantages to eliminate the diastolic runoff and increase the diastolic pressure with proved coronary perfusion pressure. Potential disadvantages include a negative effect on right ventricular function and ventricular arrhythmias due to the ventriculotomy, impaired growth of the pulmonary arteries and the need for an earlier stage II palliation. The hybrid approach (figure 4c) uses surgical branch pulmonary artery banding combined with transcatheter ductal stenting and a creation of a reliable atrial communication (balloon dilatation and stenting). The result is anatomy that achieves the goals of stage 1 palliation without the need of CPB and deep hypothermia.